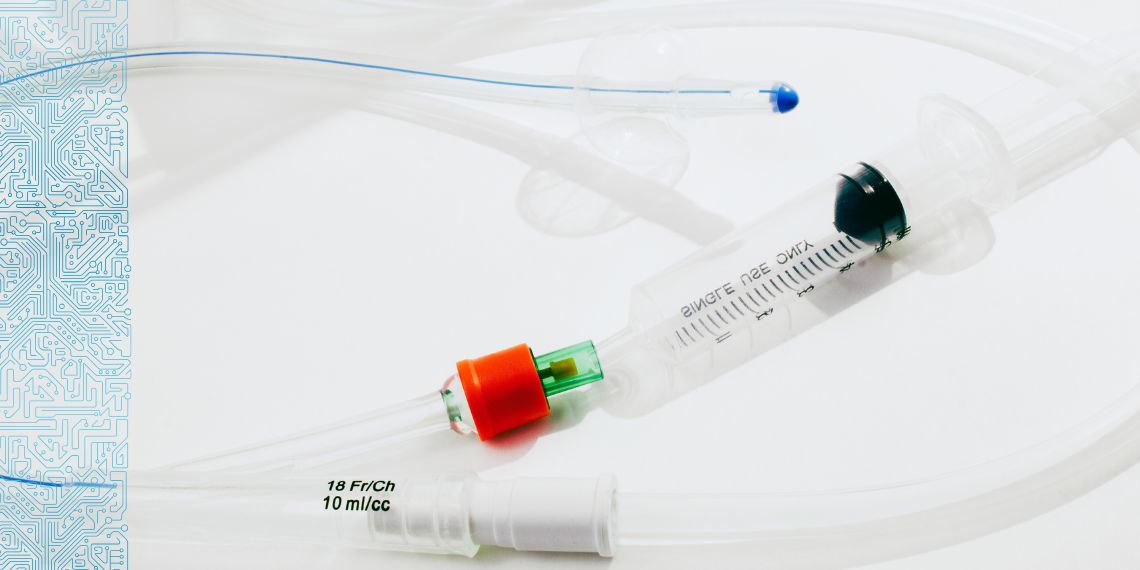
What if we could replace chemical methods of catheter sterilisation? INESC TEC is part of a multidisciplinary team that developed a medical technology set to revolutionise the way catheters are sterilised, using a solution based on infrared radiation guided by optical fibre.
The idea stemmed from a collaboration with i3S and, although previous studies have explored the use of light for antimicrobial purposes, the GoCap technology represents a significant leap forward in the field of photonics applied to healthcare. According to Orlando Frazão, a researcher at INESC TEC, the system uses an infrared light source coupled to a waveguide that precisely directs the radiation along the catheter, allowing for effective and localised elimination of pathogens. “What makes GoCap innovative is the integration of graphene oxide – a material with unique photonic and antimicrobial properties – into the waveguide, allowing a non-chemical, reusable, and safe alternative that has been little explored for disinfecting invasive medical devices,” he added,
INESC TEC played a key role in designing and implementing the electronic control system for the light source and in optimising the propagation of light through the waveguide. “We carried out lab tests like thermal trials to ensure, for instance, that the temperature reaches safe and effective levels for disinfection,” explained Orlando Frazão.
Despite the technological success, the researchers faced significant challenges. The first major obstacle was identifying a light source powerful enough to ensure the desired thermal effect. Initially, LEDs were considered due to their energy efficiency and low cost, but it became clear that they lacked the capacity to generate the necessary heat for disinfection. The solution involved adopting infrared laser diodes, which offer greater power and control.
Another critical challenge was miniaturising the system, particularly due to high energy consumption. “Using two laser diodes requires a robust power source, making the reduction of the battery’s size and weight a key challenge, since it’s essential to ensure autonomy, portability, and thermal safety in a compact format,” the researcher clarified.
The technology has already been validated under clinically relevant conditions, specifically on commercially available haemodialysis catheters and using clinical isolates obtained from infected haemodialysis catheters.
The researcher mentioned in this news piece is associated with INESC TEC

 News, current topics, curiosities and so much more about INESC TEC and its community!
News, current topics, curiosities and so much more about INESC TEC and its community!